Posts Tagged ‘embryos’
The Embryo Bank Dilemma: Reviewing the Issues, Historical Perspectives and Offering Potential Solutions

Craig R. Sweet, M.D
Reproductive Endocrinologist
Practice and Medical Director
Embryo Donation International
Introduction
In my last blog, “Why Creating ‘McEmbryos’ is Just Plain Wrong,” I wrote about my concerns regarding the creation of an embryo bank at a California clinic. In this follow-up segment, I want to re-state the issues, discuss the past history of embryo banking in the U.S., provide a list of recently written thoughtful blogs on the topic, offer possible solutions to the dilemma and discuss where we should go from here.
A “Reader’s Digest” version
California Conceptions (CC), as outlined in Alan Zarembo’s L.A. Times article apparently combined donor eggs with donor sperm and divided the resulting embryos among a number of embryo recipients. This process is commonly called a “split or shared donor/donor cycle” but was called “embryo donation” by CC. Any embryos remaining, after the recipients received their allotment, would be cryopreserved and owned by CC.
The road was paved with good intentions
I feel that CC was really trying to offer a cost-affective alternative for patients and that its true intent was to keep the size of its embryo bank as small as possible. Even with good intentions, however, it is quite likely that the embryo bank will grow. In addition, to sanction the creation of a small embryo bank will almost certainly result in the creation of larger embryo banks across the country. These banked embryos for commercial use are what I called “McEmbryos.” There also needs to be a clear distinction between embryo banking for commercial use and the process of banking one’s own embryos (i.e., collecting through multiple IVF retrievals) to be used by individuals to build their families in the future.
I still have three main concerns:
- I do not feel that embryo banks are appropriate and could result in a plethora of unintended consequences.
- I feel that corporations, businesses or physician practices should not own embryos.
- Lastly, the process of a “split or shared donor/donor cycle” should be called “embryo creation” or, at the very least, not called embryo donation.
This has happened before
An article by Gina Kolata in the New York Times in 1997 revealed that “ready-made embryos” were already being made for “adoption.” Columbia-Presbyterian and Reproductive Biology Associates were named in the article as providing “premade” embryos to patients. According to the article, most of the embryos were created when donor egg recipients backed out of the process, but the egg donors still underwent the egg retrieval; their subsequent retrieved donor oocytes were combined with donor sperm. Lori B. Andrews, a professor of law at Chicago-Kent College of Law, was quoted as having concerns about the supermarket approach to embryos while the clinicians thought it wasteful to not retrieve and fertilize the donor oocytes if the egg donors were ready for the retrieval.
In 2007, Center for Genetics and Society Senior Fellow and UC Hastings Law Professor Osagie Obasogie wrote an op-ed for the Boston Globe about a Texas center that had created an embryo bank. He was concerned about the “Wal-Martization” of human embryos, a phrase similar to my “McEmbryos.”
In November of 2012, in response to the N.Y. Times article, Jessica Cussins of the Center for Genetics and Society wrote an excellent blog on the topic, also following-up on the 2007 article by Professor Obasogie. The Texas center was eventually closed and was the subject of an FDA investigation, which eventually found that the creation of an embryo bank did not fall under FDA jurisdiction. John Robertson, Esq., wrote an excellent commentary on the topic in the Bioethics Forum in that same year.
Embryo banks have come and gone, garnering media attention and criticism and I believe it is finally time to set some ethical standards of care about them.
How New York decided to handle the embryo bank issue
 Almost five years ago, the state of New York issued regulations for tissue banks and nontransplant anatomic banks, addressing the potential of creating embryo banks:
Almost five years ago, the state of New York issued regulations for tissue banks and nontransplant anatomic banks, addressing the potential of creating embryo banks:
Embryos shall not be created for donation by fertilizing donor oocytes with donor semen, except at the request of a specific patient who intends to use such embryos for her own treatment. [NYS 52-8.7(h)]
Embryos were not to be created to store in embryo banks but only created at the behest of a specific patient and subsequently owned by that patient. Simply modifying the statement above to include “… such embryos for his/her own treatment,” would address the issue adequately, with the sentence potentially used by various organizations as they hopefully set ethical standards of care.
Potential consequences to the creation of an embryo bank
I have been called an alarmist by some for bringing up what I feel are the following potential dangers of having embryos banks in the U.S:
- If a small embryo bank is allowed to flourish, then large embryo banks will most certainly follow.
- Poorly designed and reactive legislation may be created on the state or national level as there may be further calls to regulate what are perceived to be “unregulated IVF facilities.”
- “Personhood” advocates may become further emboldened to win personhood for the embryos to protect them from becoming “McEmbryos.”
I don’t think these unintended consequences are that farfetched and need to be considered carefully should embryo banks continue unchecked.
My reluctant decision to come forward
 About the last thing I wanted to do was to comment on another reproductive endocrine practice comprised of caring staff members dedicated to the care of their patients. I have been criticized for taking such a stand and accused of doing this purely for competitive reasons. In reality, I have been working with the American Society for Reproductive Medicine’s (ASRM) and the Society for Assisted Reproductive Technologies, (SART) since October of 2011, trying to elicit a set of guidelines prior to the writing of my blog. I far preferred to stay out of the limelight and let the “powers-that-be” decide what should be done next. When the L.A. Times article was published, it de-emphasized the ethical issues and potential unintended consequences of the CC embryo banking practice, so I felt I had no choice but to bring the topic up front and center.
About the last thing I wanted to do was to comment on another reproductive endocrine practice comprised of caring staff members dedicated to the care of their patients. I have been criticized for taking such a stand and accused of doing this purely for competitive reasons. In reality, I have been working with the American Society for Reproductive Medicine’s (ASRM) and the Society for Assisted Reproductive Technologies, (SART) since October of 2011, trying to elicit a set of guidelines prior to the writing of my blog. I far preferred to stay out of the limelight and let the “powers-that-be” decide what should be done next. When the L.A. Times article was published, it de-emphasized the ethical issues and potential unintended consequences of the CC embryo banking practice, so I felt I had no choice but to bring the topic up front and center.
Others responded to the discussion
Several other infertility professionals discussed the ethical issue in articles or blogs in the weeks following the L.A. Times piece. Excluding those that simply summarized the situation, I listed below what I think are some of the better blogs:
Supporting embryo banking
- Marni Soupcoff, Esq., “Marni Soupcoff on the sale of fertilized embryos: How much for the blastocyst in the window?”
Neutral to embryo banking
- Julie Shapiro, Esq., “Custom Made or Off The Rack?”
- Carole C. Wegner, Ph.D., “Embryos for Donation: Where are the ethical boundaries?“
- Elizabeth Swire Falker, Esq., “The Bizarre World of Embryo Banking. Where My Motherhood and Morality Meet”
Against embryo banking
- Andrew Vorzimer, Esq., “Get Pregnant With Built On Spec Embryos Or Get Your Money Back!”
- Jessica Cussins, B.A., “Embryos for Sale: ‘When You Want Them, How You Want Them, or Your Money Back”
- Sara R. Cohen, LL.B., “It’s not about the Money: Why we are So Concerned about a California IVF Clinic’s Anonymous Embryo Program”
- Mikki Morrissette, “Creating Embryos To Sell“
My thanks to all of the authors for taking the time to discuss the issue in an open forum.
Proposed remedies to the current dilemma
From the beginning, I have been offering remedies to the embryo bank dilemma. Although far be it from me to tell CC how to run its business, these are a few ideas I had to offer:
Only patients should own embryos-
No organization, corporation or physician practice should own embryos except in the most extreme circumstances, such as embryo abandonment. With embryo donation, it is most appropriate that the donor facility simply holds the embryos, with the donors still being able to request the return of their embryos, up to the point of transfer into the recipients, should a catastrophic occurrence take place, Attorneys refer to this as being a guardian, a conservator, or a temporary holder of goods. When presenting at the American Bar Association Family Law Section Spring conference in April of 2012, many of the attorneys there strongly supported the concept of conservatorship of the donated embryos over facility ownership.
If the embryos are returned to the donor, it seems appropriate to ask the donors to reimburse the embryo donation facility for all reasonable fees expended in originally obtaining the donated embryos and returning them to the donors. We have been running our embryo donation program this way for over 12 years and we encourage others to do the same.
Excess cryopreserved embryos could be owned by patients-
As best as I can surmise for CC, their business model is to recruit a number of embryo recipients and then transfer 1-2 donor/donor embryos into each recipient. I suggest that any remaining embryos be owned by one or more of the recipients and the entire cycle should not move forward until at least one patient agrees to take the extra cryopreserved embryos, should any exist. Extra charges could be levied to those that secure the remaining embryos. In this way, no embryos remain to create an embryo bank and the CC business model remains essentially intact.
Renaming the process-
The combination of donor sperm with donor eggs and then calling them donated embryos does not fit with the ASRM definition of embryo donation (Ethics Committee of the ASRM, 2009). Embryo creation is a far better term or “shared or split donor/donor cycle” is perhaps even more appropriate. Calling such embryos donated embryos debases the amazing gift that embryo donors provide when donating their embryos.
Who should set the standards?
 SART has reviewed the concerns stated in my previous blog but I don’t think it yet has arrived at a
SART has reviewed the concerns stated in my previous blog but I don’t think it yet has arrived at a
conclusion. My understanding is that the ASRM Ethics Committee is to take up the topic during the early months of 2013. As our main guiding societies, I believe they need to take the lead, develop position statements and provide ethical standard of care guidelines for all practices to use.
Once ASRM and SART have provided ethical standard of care guidelines, I will next request that the

Canadian Fertility and Andrology Society and the European Society for Human Reproduction and Embryology (I am a member of both) consider the topic and respond with their own recommendations if they see fit.
It is not out of the realm of possibility that numerous societies could collaborate to form a consensus, such as they did when they banned the support and publication of human reproductive cloning research.
Summary comments
So where are we now on this dilemma? SART has discussed the topic but summary statements are pending. The ASRM Ethics Committee will soon meet, with the embryo bank topic apparently on the agenda. Assuming the Ethics Committee feels the topic has merit, I am uncertain how long it will take for them to release a position statement. I am hopeful that “the powers that be” will be attentive in finding a compromise that will allow CC to continue to offer their skilled reproductive services while preventing the formation of an embryo bank, no matter the size, further clarifying who should own embryos as well as the definition of embryo donation as it pertains to the current situation.
I don’t know about you but I don’t really like the idea of “McEmbryos,” or the commodification and “Wal-Martization” of human embryos. Patients should own them and decide their destiny. I am hopeful that our guiding societies will do just that – guide us on this sensitive and important topic.
Special thanks:
Thanks to Grace Centola, Ph.D., for helping to find the New York State statutes pertaining to embryo banking.
Thank you to Jessica Cussins for her blog on the topic, the reference by Professor Obasogie and her followup on the now closed Abraham Center for Life.
References:
Ethics Committee of the American Society for Reproductive Medicine. American Society for Reproductive Medicine: defining embryo donation. Fertil Steril. 2009 Dec;92(6):1818-9
.
Why Creating “McEmbryos” is Just Plain Wrong
Introduction
Recently, Alan Zarembo of the L.A. Times released a story alleging that California Conceptions was combining donated eggs with donated sperm and called them “donated embryos.” If there were leftover cryopreserved embryos, ownership of the embryos apparently went to California Cryobank (CC). If true, this is an egregious assault on reproductive ethics.
Defining embryo donation

About one-third of all patients undergoing in vitro fertilization (IVF) will have excess embryos to cryopreserve. About one-half of these will not be used for reproduction by the patients who created them. (Bangsboll et al., 2004; Lyerly et al., 2010) Embryo donation occurs when patients with unused cryopreserved embryos make the amazing decision to pay it forward and donate their embryos to patients in need. In true embryo donation, the patients own the embryos and make disposition decisions regarding their embryos. It is estimated that 2-10% of all cryopreserved embryos are donated to patients in need.
Why is creating “McEmbryos” so wrong?
Allegedly, California Conceptions would use donor sperm and donor eggs to create embryos. At times, all the resulting embryos would be transferred simultaneously to numerous couples, each receiving approximately two embryos at a time. This would commonly be called a “split donor/donor cycle.” It is, however, an absolute misrepresentation to call them donated embryos. At least in this split donor/donor cycle scenario, where all the embryos were transferred, there would be no cryopreserved embryos left whose ownership was uncertain.
What if not all the embryos were transferred and some were cryopreserved? As I understand, CC transferred no more than two embryos at a time to numerous patients, with the remainder cryopreserved. The residual cryopreserved embryos became the property of CC.

According to the article, egg and sperm donor profiles are sent to prospective recipients. As soon as CC received a “buy-in” from a few patients, the “donor embryos” were created from the donor eggs and sperm. Who made the decision to combine these two donors? What happens to the left over cryopreserved embryos? The article stated that dozens of embryos would be created through the combination of a pair of donors with the embryos then frozen while CC looked for patients who wanted them. And yet, the clinic claimed to have only ten sets of cryopreserved “donated embryos” in their tanks at any given time.
While they stated they didn’t want to create a bank, it would appear that this is precisely what was being done.
I feel there is a potential conflict of interest on the part of any IVF facility regarding the disposition decision of any cryopreserved embryos that they own. Patients normally have options such as using them to build their family, donation to science, donation to a laboratory for quality assurance testing and personnel training, keeping them cryopreserved forever, and finally, donating them to patients in need (embryo donation).
With the methods described in the article, a number of logistical and ethical questions arise:
- Were the sperm and egg donors fully aware of what was to be done with the resulting embryos?
- How did the IVF practice decide which sperm and egg donors should be combined? Was this decision made through market research? If so, who made these decisions?
- Is anyone tracking where all these donor-donor conceived offspring end up?
- Who ultimately owns the cryopreserved embryos? In the article, the physician interviewed said the clinic owned them when they were frozen
- Who makes the disposition decisions regarding these embryos? Will the IVF facility that owns the embryos be likely to make disposition decisions that do not benefit their bottom line? Who has the best interests of the cryopreserved embryos at heart when the embryos are owned by the IVF facility? Is it possible that CC is the entity that is really “donating” their remaining cryopreserved embryos
- What happens to the cryopreserved embryos if the practice closes or is sold?
- Perhaps most important, although it may not be a problem presently but for the future, what happens to the cryopreserved embryos that are never chosen?
While certainly not meaning to demean the cryopreserved embryos, I can’t help but think of these dono/donor cryopreserved embryos as fast food embryos or “McEmbryos.” I can just see the patients coming in, looking above the cash register to the menu above and ordering a “Number 3,” which has a burger (i.e., bright, blonde-haired, blue-eyed egg donor) and fries (i.e., handsome, athletic and tall sperm donor) and ends up getting the order supersized to boot (i.e., requesting twins). I should write clearly that this is not the current method CC uses to to match recipients to remaining cryopreserved embryos but the slippery slope exists and other practices may eventually emulate this process
What did EDI try to do over the past year to remedy the problem?
We suspected what might be happening at CC well over a year ago but didn’t have proof. We approached the American Society for Reproductive Medicine (ASRM) who examined the information and eventually sent it on to the Society for Assisted Reproductive Technologies (SART). SART did an investigation agreeing with our preliminary assessment. Unfortunately, CC was not a member of SART so they had little influence with CC.
There was some discussion of placing CC on the Center for Disease and Control’s (CDC) radar, asking that they potentially audit CC. I am not aware if this was ever done.
There was some discussion of revising the Ethic’s Committee Opinion on embryo donation detailing the inappropriateness of creating “McEmbryos,” but in the recent revision sent out to all ASRM members to review this past August contained no such language. I contacted ASRM and volunteered to help write the couple of paragraphs that would address the issue but I was never contacted. My current understanding is that the ASRM Ethics Committee will be taking up this issue in the beginning of 2013 and I am hopeful they will make a stand against creating “McEmbryos.”
Is it really ASRM or SART’s fault?
ASRM and SART are membership organizations. Anyone can join ASRM whereas SART is only open to IVF practices. These entities are not designed to truly police their members. Certainly, they can bar them from membership, but let’s face it, this is really not much of a punishment. Most patients are not really aware if a practice is a member of either organization, so expulsion does more to protect ASRM and SART from criticism than it does to protect the patients.
In reality, I am not suggesting that ASRM or SART begin sanctioning their members. These organizations are made up of bright individuals wanting what is best for members and patients alike. I don’t believe ASRM or SART could ever expose themselves to legal liability and try to be anything more than what they were originally designed to be.
I feel criticism towards ASRM and/or SART is potentially misplaced. While I do feel these organizations can certainly take a stand against the creation of “McEmbryos,” they can only provide educational materials and information to members and patients. never entering the realm of fining, condemning practices or policing practices. That is simply not their job.
I am not being insensitive to the needs of the patients
California Conceptions is providing amazingly healthy embryos to patients in need. How could anyone not be touched the story twin girls born to a single 41 year-old woman? There is no question that the donor/donor split cycle can be far more cost effective than many other options. The ends to this process are what we all strive for – a healthy family.
Call me old-fashioned but I still feel there are instances where the ends cannot justify the means. Are we are loosing all respect for the embryos and treating them utterly as a commodity? The slippery slope is becoming quite steep. Are we ready for expanding banks of unclaimed embryos across the country owned by practices or physicians and not the patients? I’m sorry but the means being used here are fraught with uncertainty and may result in a list of unintended consequences.
What are the potential repercussions of the L.A. Times story?

At least this election year is over. I had grave concerns that the story would break early in the election cycle and would become a political football, resulting in a series of consequences around the country. I still believe we are at risk in the following ways:
- • Poorly designed and reactive legislation may yet be created on state or national level
- • This story may motivate the “Personhood” advocates by encouraging them to win personhood for embryos thereby protect them from becoming “McEmbryos”
- • There will be “guilt by association,” leaving other legitimate embryo donation facilities open to criticism and ridicule
- • All of this will reflect poorly on basic IVF facilities that are frequently viewed as unregulated, even though we are accountable to more regulatory agencies than any other area of medicine
There may be significant backlash regarding this story and we should all be prepared to answer questions that may arise from the media, our peers and our patients.
How can embryo donation programs hold but not own the donated embryos?
At Embryo Donation International (EDI), the embryo donors are still able to request that their donated embryos be returned if needed. For example, if the children of an embryo donor were tragically killed in a motor vehicle accident, it seems absolutely appropriate that the cryopreserved donated embryos should be sent back to the donors. To discourage this from being done without good reason, transportation costs for returning the embryos, which is estimated to be $300, are the responsibility of the original donors.
Once the embryos are transferred into the recipients, however, they now “own” them. In this way, EDI never truly owns the donated embryos. We are the conservator and protect the embryos while never making any disposition decisions regarding their fate. We never own the donated embryos and we encourage all embryo donation facilities adopt this model of conservatorship.
What can be done to correct the current problem?
- Various membership organizations such as ARSM, Pacific Coast Fertility Society (PCFS), SART and RESOLVE could release position statements condemning the creation of “donated embryos” without a destination. This could help guide public opinion.
- Perhaps the embryo donation programs would be willing to sign a contract stating that they will not participate in the creation of embryos without a destination. Programs willing to sign and honor the contract would be adhering to the highest of ethical standards.
- Through the court of public opinion and if the L.A. Times article is accurate, patients may no longer want to participate in what they feel in an unethical practice.
- Legislative action on the part of California may be necessary but this will take time and a great deal of expertise. We run the risk, however, of having well-meaning legislative actions spilling over and harming the process of embryo donation or IVF itself.
- CC itself may need to modify its business model by allowing patients to own the cryopreserved embryos.
From my perspective, we have a few options:
Something really needs to be done. I don’t feel that this unethical practice should continue. A corporation, business or physician practice should never own embryos, no matter how brief. These embryos are also not truly donated unless you feel CC is able to donate the excess cryopreserved embryos. The question is if we should play an active or a passive roll in determining what takes place. I personally prefer the active roll so you can count EDI in on doing our best to guide the process.
Sour grapes? Nope, just sour taste!
CC is a potential competitor of EDI. I have made this clear to each and every person I have spoken to regarding this current dilemma. When we were contemplating the expansion of our ten-year embryo donation program into EDI, we also looked at the option of a money-back guarantee such as is offered by CC. Understanding the delivery rates for recipients of truly donated embryos ranges from 27-45%, the likelihood of returning the majority of the payments back to recipients made it unlikely we could even keep our doors open. Most “shared risk” options also charge a premium on top of the normal fees. Recalling that embryo recipients are emotionally and financially drained, the idea of tacking on a large additional fee seemed unfair to the recipients and quite impractical to the process. Embryo donation works for recipients, in part, because it is more cost-effective than many other options.
So you see, it is not “sour grapes” that is guiding my writing; it is just a sour taste. If the article was accurate, I feel strongly that creating “McEmbryos” is an affront to IVF and embryo donation programs, misleading to embryo recipients and totally unfair to the cryopreserved embryos that are currently waiting to be chosen.
If you agree, please co-sign this blog….
We are going to do something a bit different with this blog. We are strongly encouraging not just the routine comments that follow our blog but we are also asking that practices, patients and interested parties who agree that creating “McEmbryos” is ethically inappropriate sign below. By endorsing the statement below, we will hopefully begin to separate ourselves from such entities that practice the “McEmbryo” method of “embryo donation,” moving forward with a resolution that will work best for all.
routine comments that follow our blog but we are also asking that practices, patients and interested parties who agree that creating “McEmbryos” is ethically inappropriate sign below. By endorsing the statement below, we will hopefully begin to separate ourselves from such entities that practice the “McEmbryo” method of “embryo donation,” moving forward with a resolution that will work best for all.
By signing below, I do hereby agree that creating banks of cryopreserved embryos is ethically unjustifiable and support sound solutions that will adhere to the highest of ethical standards supporting patients and cryopreserved embryos in the best possible way.
References:
What Impacts Embryo Donation Success Rates?

Craig R. Sweet, M.D.
Reproductive Endocrinologist

Corey Burke, B.S., C.L.S.
Laboratory Supervisor
Introduction
Asking what determines the success rates in embryo donation is an excellent question. The answer, as one might expect, is neither simple nor completely understood.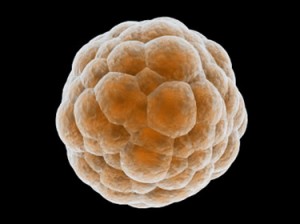
Embryologists and physicians try to choose the fewest number of healthy embryos for fresh transfers to increase success rates while minimizing multiple pregnancy rates. It is indeed a delicate balance. For example, it is well understood that in Europe, physicians transfer fewer embryos but patients also suffer significantly lower success rates than in North America. (Boostanfar R, et al. Fertil Steril 2012)
What variables do we examine to estimate probable success?
Since donated embryos are cryopreserved, the variables become even more complex compared to fresh embryos. In combining a great deal of published data and over 20 years of IVF and embryo transfer experience, we came up with what we feel are the variables which seem to influence success rates:
| Very Important! |
Preferred |
Less Optimal |
| Did the fresh cycle in which the embryos were frozen result in a pregnancy & delivery? |
Successful pregnancy and delivery |
Fresh transfer resulted in miscarriage or no pregnancy |
| Number of embryos available in a given donated set |
Four or more |
Three or fewer |
| Past implantation rates of both fresh and frozen embryo transfers |
High implantation rates |
Low implantation rates |
| Quality of the embryos frozen (link) |
High quality (Blastocyst) |
Medium quality |
| Age of the women when the eggs were provided to create embryos |
Less than 35 years old |
35 years of age or older |
| Overall health of the embryo recipient (link) |
Healthy |
With treated or untreated medical issues |
| Important |
Preferred |
Less Optimal |
| Stage of growth when the embryos were frozen (link) |
Day 5, blastocyst stage |
Day 3, 8-cell stage |
| Technique used to freeze/thaw or vitrify/warm the embryos (link) |
Vitrification |
Slow freeze |
| Overall frozen embryo transfer pregnancy rates for facility freezing the embryos |
30% or more |
Less than 30% |
| Overall frozen embryo transfer pregnancy rates for facility thawing the embryos (EDI) |
High at 30% or more |
Less than 30% |
| Ejaculated vs. surgically aspirated sperm used for fertilization |
Ejaculated sperm |
Surgically aspiration sperm |
| Somewhat Important |
Preferred |
Less Optimal |
| Was preimplantation genetic testing of the embryos done? |
Yes |
No |
| Age of the male producing the sperm |
Less than 40 years old |
More than 40 years old |
| Past successful deliveries with other embryos from donating facility |
Yes, with past deliveries |
Miscarriage, reduced survival of embryos or failed implantation |
| Probably Unimportant |
Preferred? |
Less Optimal? |
| Cryopreservation duration |
Less than 10 years? |
More than 10 years? |
The above variables influence EDI’s decision to both accept embryos from other facilities as well as determine how many donated embryos should be thawed/warmed to achieve a successful delivery.
The importance of the embryo recipient’s health should not be underestimated
In a previous blog, we described how important it was for our embryo recipients to be healthy. (link) Inadequately treated health problems, harmful medications, recreational drug use as well as smoking and weight concerns all play a potential role affecting success rates. It is clear that success depends on both the quality of the donated embryos and the overall health of the recipient.
Are all donating IVF facilities the same?
We understand that not all IVF facilities have the same success rates. Some facilities will have provided EDI  donated embryos with consistently high implantation rates while others may provide embryos of consistently lessor quality. EDI examines a facility’s past fresh and frozen embryo transfer pregnancy rates as well as its past history of providing EDI with donated embryos. It does, however, take a fair amount of time to seemingly identify a trend but we endeavor to examine all the variables we can. Our contact management database system was recently upgraded to track these variables more consistently.
donated embryos with consistently high implantation rates while others may provide embryos of consistently lessor quality. EDI examines a facility’s past fresh and frozen embryo transfer pregnancy rates as well as its past history of providing EDI with donated embryos. It does, however, take a fair amount of time to seemingly identify a trend but we endeavor to examine all the variables we can. Our contact management database system was recently upgraded to track these variables more consistently.
Do your embryos make the grade?
Understanding that all of the above variables are quite complex, we endeavored to find a simple way to convert the data into something patients could more easily understand. Since nearly all patients understand the basic A, B & C grades we used to receive in school, we modeled our grading of the embryos around these letter grades.
We created a mathematical model to assess a number of the above variables, converting the final analysis to A+, A, A-, B+, B and B- letter grades. Interestingly, we found the model really did help to predict delivery rates and continue to use it to this day to grade individual embryos as well as entire sets of donated embryos.
What information do we gain on the day of thaw and embryo transfer?
Most frequently, the embryos are thawed just hours before transfer. At times, we may thaw them days prior if, for example, they were frozen early in their development and we want to grow them further before deciding how many to transfer. Therefore, the following last set of variables will also influence success rates:
| Important |
Preferred |
Less Optimal |
| Survival rates of thawed embryos |
100% |
Less than 100% |
| Overall appearance of the thawed embryos |
Healthy, expanding and growing |
Evidence of cellular damage |
If 100% of the embryos, perhaps three out of three, survive the thaw and look healthy, we feel this is a good sign. If only 50% survive, for example perhaps only two of four, then we are concerned that the overall implantation rates will be reduced and that we might need to find more embryos to transfer just to get to the “finish line” of pregnancy and delivery. Ultimately, we want at least two high-quality embryos or up to four less certain quality embryos placed on the day of transfer.
What are the national success frozen embryo transfer delivery rates?
 In 2009, the CDC reported that there were 26,069 frozen embryo transfers performed in the U.S. with an average delivery rate of 31% per embryo transfer procedure. In addition, there were 6,074 frozen embryo transfers using embryos created from donated eggs (i.e., younger women) with slightly higher delivery rates of 34%. Please recall that donated embryos generally come from the very same types of patients listed in these success rates.
In 2009, the CDC reported that there were 26,069 frozen embryo transfers performed in the U.S. with an average delivery rate of 31% per embryo transfer procedure. In addition, there were 6,074 frozen embryo transfers using embryos created from donated eggs (i.e., younger women) with slightly higher delivery rates of 34%. Please recall that donated embryos generally come from the very same types of patients listed in these success rates.
What are EDI’s success rates?
We do our best to screen the embryos, carefully trying to choose the embryos most likely to implant. We estimate delivery rates with donated embryos to range from 27 – 42%, depending on the many variables listed in this blog, with a multiple pregnancy rates of 20-25%. We wish the success rates were higher, but please understand that frozen/thawed embryos implant and grow less frequently than fresh embryos and these percentages are entirely consistent with the frozen embryo transfer success rates described in 2009, which ranged from 31-34%.
Even with the slightly lower delivery rates compared to fresh embryo transfers, embryo donation remains one of the best and most cost-effective options for patients who cannot otherwise afford egg donation or qualify for adoption. Embryo donation still allows a woman to experience pregnancy and delivery while bonding, nurturing and protecting the ongoing gestation.
Summary
There are many variables that go into determining the potential success rates for a given set of donated embryos. First, we attempt to examine these variables carefully in deciding if EDI will accept the donated embryos. Second, we use this same information to determine how many embryos we should thaw/warm and eventually transfer. The process remains a bit of an ART (pun intended) since the complete understanding of how all of these variables influence each other and the ultimate success rates are yet to be fully known.
References
“Annual ART Success Rates.” Centers for Disease Control and Prevention. Division of Reproductive Health, 19 Apr. 2012. Web. 24 Apr. 2012. <http://www.cdc.gov/art/ARTReports.htm>.
Boostanfar R, Mannaerts B, Pang S, Fernandez-Sanchez M, Witjes H, Devroey P; Engage Investigators. A comparison of live birth rates and cumulative ongoing pregnancy rates between Europe and North America after ovarian stimulation with corifollitropin alfa or recombinant follicle-stimulating hormone. Fertil Steril. 2012 Mar 27.
Reasons to Donate
By: Vicki D., an embryo donor
“In the order of nature we cannot render benefits to those from whom we receive them, or only seldom. But the benefit we receive must be rendered again, line for line, deed for deed, cent for cent, to somebody.’ – Ralph Waldo Emerson, Compensation
On May 13, 2010, I made a decision. It wasn’t your normal everyday decision on things like what shoes to wear, where to have lunch, or which shade of lipstick to buy. It even surpassed those important decisions we encounter in life such as what house to buy, the most lucrative financial investments and selecting the best care for elderly parents. It was a decision much more substantial, incredibly emotional and most importantly, everlasting. It was regarding the fate of our embryos. Yes; five embryos, frozen, suspended in time – a significant and extraordinary reminder of a successful IVF cycle producing twin girls just two years prior. Considering that my family was now complete, the desire to have more children had abated. But the process was far from over and I knew this going in. There are five potential lives to consider currently residing in a sub-zero environment. So the question remained… what did one do with extra embryos when one’s need or desire to expand your family has subsided?
In my quest to determine the future of my five frozen embryos, I discovered several options to choose from. These ranged from permanent storage – or in some cases “abandonment,” destruction, donation to stem-cell research,  donation to the IVF clinic lab, or donation to an infertile couple or person in need. Continued voluntary storage brought unnecessary substantial fees, not to mention the inevitable procrastination of decision making. Abandonment wasn’t an option for obvious reasons as I felt a responsibility towards these embryos. The only things I have ever abandoned in my life were the occasional art project or my first premature marriage in my early twenties. The concept of destruction simply didn’t make logical sense. Why go through all the expense and trouble of creating embryos that one day would be destroyed because there was no better option considered? Stem-cell research or IVF clinic donation seemed like fair choices since I wouldn’t have my twins without past research in IVF. Even so, there had to be a better option available that would help promote the preservation of life and help out infertile couples desperately wanting a baby.
donation to the IVF clinic lab, or donation to an infertile couple or person in need. Continued voluntary storage brought unnecessary substantial fees, not to mention the inevitable procrastination of decision making. Abandonment wasn’t an option for obvious reasons as I felt a responsibility towards these embryos. The only things I have ever abandoned in my life were the occasional art project or my first premature marriage in my early twenties. The concept of destruction simply didn’t make logical sense. Why go through all the expense and trouble of creating embryos that one day would be destroyed because there was no better option considered? Stem-cell research or IVF clinic donation seemed like fair choices since I wouldn’t have my twins without past research in IVF. Even so, there had to be a better option available that would help promote the preservation of life and help out infertile couples desperately wanting a baby.
I remember the years of anguish I experienced being infertile. Everywhere I went I saw pregnant women or newborn babies. It became an obsession, perceived as something so intangible for me yet came so easily for others. There would be no remedy but a child. Why not help another couple expand their family? Why not help end the anguish? Why not “Pay it Forward” to the infertile community in such desperate need? The simplest answers to these questions became the best option.
So the decision came with three stages. Firstly, there was the genetic hurdle to consider. There is something about setting your genetic code, or more specifically, your potential genetic offspring, free into the world that can be somewhat unsettling. Where will these embryos end up, will they survive and what kind of life will they have? Will they know their history? Will they have questions? Will they look like me? But in the greater scheme of things, do these questions really matter?
The value and definition of family transcends any DNA makeup.
In the pursuit of the family unit, we tend to look beyond the genetic code and focus on the family element. The concept of having a family does not automatically equate to comparable genetic material as can be seen with any family with an adopted child. It’s about being part of a team, functioning as a whole and sharing your love and commitment to live and experience life together.
Secondly, there is an element of giving back; Paying it Forward to the infertile world. Donating the embryos was my way of paying back what I was so very fortunate to finally have – a family. Prominent memories of being unfulfilled without children are still fresh in my mind. I honestly believe that donating my idle embryos to someone in need helps to promote the probability of life.
And finally, chance. The chance to help someone build a family, the chance of potential life for the embryo, the  chance for you to give the greatest gift in life, the chance to take a chance! So for any of you out there who find yourselves with important decisions to make about your embryos in storage, think back a bit to your own infertile days. That memory will help guide you to do the right thing for someone with the same needs and desires as you have. Take a chance and do something good for humanity.
chance for you to give the greatest gift in life, the chance to take a chance! So for any of you out there who find yourselves with important decisions to make about your embryos in storage, think back a bit to your own infertile days. That memory will help guide you to do the right thing for someone with the same needs and desires as you have. Take a chance and do something good for humanity.
Donating the embryos was my way of paying back what I was so very fortunate to finally have – a family.
In the end I trusted Embryo Donation International (EDI) and Dr. Sweet with my precious embryos. I knew that with their high ethical standards and sheer devotion to the embryo they would give my embryos a chance at life, and hopefully help to build another family, just like they helped to build mine. And on every Mother’s Day ever since the donation I hope and wonder that by liberating my embryos they were able to help create another family somewhere out there and that they are as happy as I am.
Vicki D.
Mom, a Loving Wife and now, an Embryo Donor
torig71@gmail.com
How Does EDI Decide to Exclude Potential Embryo Recipients?

By: Craig R. Sweet, M.D.
Reproductive Endocrinologist
Info@EmbryoDonation.com
Introduction
There are times when we need to exclude patients when they contact Embryo Donation International (EDI) to become an embryo recipient. While not an easy decision or discussion, I thought it was time to explain our rationale when needing to, at least temporarily, exclude potential embryo recipients.
In a way, the blog we recently wrote on the ranking of potential embryo recipients dovetails into this discussion. In this blog, we described what we thought was an appropriate ranking system prioritizing those patients with the greatest need.
Our discussion here focuses on the patients who apply but who are never ranked because EDI feels they should be excluded because of any number of the reasons described below.
Excluding Potential Embryo Recipients Due to Maternal Risks
The decision to exclude a patient from embryo donation is really, in some ways, no different than the decision we have to make with other infertility patients.
Relative contraindications to pregnancy
There are occasions when certain conditions should probably be corrected before pregnancy takes place since pregnancy will often worsen or complicate the condition. Examples may include gallbladder disease and ovarian cysts or surface uterine fibroids that are five+ centimeters in average diameter. Treating these problems once pregnancy is established is very difficult, so it may be best to control the situation while we still can and correct the potential problem first before conception.
Strong contraindications to pregnancy
Patients who would be at significant risk of illness or even death should pregnancy occur include those suffering from cancer, poorly controlled systemic lupus, pulmonary hypertension or diabetes, to name a few important disease states.
Infrequently we have to be a bit paternalistic and say “no,” understanding that we may cause great harm to our ill patients by assisting them to become pregnant.
Excluding Potential Embryo Recipients Due to Risks to the Embryo/Fetus
The trickier decisions involve those patients where the potential for delivery of a live child is measurably reduced. Patients with decreased embryo implantation rates and those who are at an increased risk for spontaneous loss or at an increased risk for premature delivery/stillbirths fall into these categories.
Some of these examples are listed below:
|
Situation |
Decreased implantation |
Increased risk of spontaneous loss |
Increased risk of significant prematurity or stillbirth |
| Uterine cavity distorting fibroids or polyps |
x |
x | |
| Uterine fibroids 2+ cm in size located within the uterine muscle |
x |
x |
|
| Damaged uterine cavity with a thin endometrial lining |
x |
x |
|
| Hydrosalpinx where tubal fluid may flow back into the uterus |
x |
x |
|
| Unexplained recurrent pregnancy loss |
x |
||
| Uncontrolled medical conditions (e.g., hypertension, diabetes, renal disease, autoimmune disease) |
x |
x |
|
| History of an incompetent cervix |
x |
x |
|
| Persistent history of premature births |
x |
||
| Untreated pre-diabetes |
x |
x |
x |
| Obesity or morbid obesity* |
x* |
x* |
x |
* The effects of obesity or morbid obesity with regards to implantation rates and spontaneous loss rates are controversial.
The “Grey” Exclusion Zones
Out of the list above, one of the most difficult categories involves those patients who are obese or morbidly obese and their  potential reduction in implantation rates and increased spontaneous loss rates. Some articles show a significant reduction in implantation rates and an increased risk of spontaneous loss while others show contradictory results. While a comprehensive review of this topic goes beyond the scope of this blog, I believe there are a few things we understand:
potential reduction in implantation rates and increased spontaneous loss rates. Some articles show a significant reduction in implantation rates and an increased risk of spontaneous loss while others show contradictory results. While a comprehensive review of this topic goes beyond the scope of this blog, I believe there are a few things we understand:
- Patients with glucose intolerance and insulin resistance, regardless of weight, are at a higher risk of developing gestational diabetes during pregnancy and the potential complications associated with this disease.
- Patients who are obese or morbidly obese are clearly at risk during pregnancy for a host of issues, including preeclampsia, hypertension, large for gestational age babies, prematurity, stillbirths, gestational diabetes, Cesarean section deliveries and the risks associated with these surgeries.
There is only one preliminary study specific to embryo donation that did not show a consistent decrease in pregnancy rates as weight increased (Body Mass Index: BMI) but the trends were present suggesting an average reduction in overall pregnancy rate of 33% for obese and morbidly obese patients with a BMI of 30 or more. (Finger R., et al. 2011) We await the detailed publication of this important study to better understand this issue.
Weight loss is terribly difficult for patients and takes a great deal of time. Sometimes surgery, such as a gastric band or intestinal bypass surgery, may be the best option. These issues are the thorniest to decide, with pressure applied by potential embryo recipients who do not fully understand how their weight may contribute to failed implantation or pregnancy loss, though it certainly places them and their unborn offspring at greater risks during the pregnancy.
Surrogacy as an Option When Material/Embryonic/Fetal Risks are Too High
 Some of the issues discussed in this blog can be treated. Then the potential embryo recipient can be moved out of the exclusion zone. Some of these issues are not treatable, so options such as surrogacy and/or adoption may be better alternatives.
Some of the issues discussed in this blog can be treated. Then the potential embryo recipient can be moved out of the exclusion zone. Some of these issues are not treatable, so options such as surrogacy and/or adoption may be better alternatives.
While some embryo donation programs refuse to allow surrogacy, EDI feels this is an excellent alternative. For example, is it ethical for EDI to ask that the patient with asymptomatic uterine fibroids, which may significantly reduce implantation or increase pregnancy loss rates, be surgically removed in a patient who fears surgery? Is gestational surrogacy a better alternative?
In Summary
We do not mean to be cruel or judgmental but are forced to make difficult decisions regarding the acceptance or exclusion of embryo recipients. We owe it to the potential embryo recipients to give them the best chance possible, understanding there are medical conditions that may severely impair their chances for success. We owe a debt of gratitude to the embryo donors and take seriously the responsibility of finding a healthy patient for their embryos hoping to maximize the chances that the embryos will survive and thrive. Lastly, and certainly not least, we owe it to the embryos to make certain they have the best chance possible.
Unfortunately, we sometimes have to make the difficult decision to exclude a potential embryo recipient, at least temporarily, until the medial concerns are remedied or certainly improved.
References:
Finger R, et. al. Obesity and the ability to achieve pregnancy in embryo donation. Fertil Steril 2011;96(3)-S172.
Parent via Egg Donation – Parent via Embryo Donation

Marna Gatlin of PVED
Exploring what we know – Marna Gatlin, Founder Parents via Egg Donation and guest blogger
I was asked recently what I thought about embryo donation vs. egg donation. Is a parent via egg donation the same as a parent via embryo donation? What do they have in common? Or are they very different?
My knee jerk reaction was “Well doh, of course they are the same. They are both embarking upon a unique journey to become parents right?”
When I thought more about it, the word “versus” jumped out at me. Did the individual asking the question really mean “versus” as in against, or in contrast to?
Are the two mutually exclusive?
When I think of egg donation and embryo donation, I think about the word “AND” – I don’t think of it as an either or. Both are just different ways of either growing or adding to your family.
Some approach embryo donation with great trepidation because they worry about the bonding process, or about the explanation or story they will be sharing with their child. The reality is – the path might be different but at the end of the day the goal is the same – you are becoming a parent.
Let’s delve a little deeper. If you receive a donor egg, the genes of your baby are going to be combined with the genes of your husband (or partner) and those of your egg donor. Or if you are a single mother, or women in a same sex relationship, the donor egg will be combined with donor sperm. You will probably undergo what we call a fresh embryo transfer. You will carry that baby for nine months and then deliver that baby. Women often wonder if there’s a down side to carrying a baby that is not genetically related to them. You know this is an age old question that’s been asked and answered since the early 80’s when the first donor egg child was conceived, carried, and delivered. I can tell you as a parent via egg donation myself that it doesn’t matter. I carried my son for nine months. The bond I created with him is rock solid, loving, and he’s my son. The lack of genetic connection simply doesn’t matter. Not one iota. The very same thing happens with embryo donation recipient parents who are receiving truly a meaningful gift of life.
genes of your husband (or partner) and those of your egg donor. Or if you are a single mother, or women in a same sex relationship, the donor egg will be combined with donor sperm. You will probably undergo what we call a fresh embryo transfer. You will carry that baby for nine months and then deliver that baby. Women often wonder if there’s a down side to carrying a baby that is not genetically related to them. You know this is an age old question that’s been asked and answered since the early 80’s when the first donor egg child was conceived, carried, and delivered. I can tell you as a parent via egg donation myself that it doesn’t matter. I carried my son for nine months. The bond I created with him is rock solid, loving, and he’s my son. The lack of genetic connection simply doesn’t matter. Not one iota. The very same thing happens with embryo donation recipient parents who are receiving truly a meaningful gift of life.
The most beautiful aspect of embryo donation to me is that embryos that are being placed for donation are done so purposely. These are embryos that the donating parents know have created amazing children who are loved, honored and cared for. These donating parents want to make sure their embryos are donated to a home that will love, cherish and honor the resulting children as they would. Regardless of whether an individual has a child via egg donation or embryo donation, the fears of parenthood almost always focus on the unknowns. And here’s a secret: they are also experienced by those who are conceived naturally. Moms and Dads all over the world have the same worries about parenting as do parents via egg donation and embryo donation.
Parents via egg donation often ask questions such as:
- Am I going to screw up my child?
- Will my child love me?
- How am I going to relate to my child?
- Will my parents and other family members accept my child?
- How and when will I share information about their conception?
When we look at embryo donation the questions that we find unique to embryo donation are:
- Are we protected legally, can the donating parents come back and claim our child in the future? (The answer is NO, they cannot. That’s why it’s important to have a clear legal contract.)
- Will my child have access to information about his or her health in the future?
- Will my child have siblings and if so, will they have the opportunity to know them?
- Will my child or children see me as their real parent?
- How will I explain this to my family or friends?
- What about stupid comments from those around me?
- How and when will I share the information about my child’s conception with my child?
To me there is no difference between being a parent via egg donation or a parent via embryo donation. The end result is the same.
At the finish line we are simply Mom and Dad.
Marna Gatlin
Parents via Egg Donation
www.PVED.org | marna(at)pved.org
A little bit about Marna…
After many years of struggling with infertility, PVED founder Marna Gatlin discovered that the technology to have a child through egg donation was available. She was curious, excited, and above all, hopeful that this process might be the conduit to finally achieving her lifelong dream of becoming a parent.
Marna ensures that all the needs of egg donor recipients are met, maintaining a high standard of ethics and confidentiality. Marna advocates and assists recipient parents, helping them arrange for the highest quality patient care, wherever in the world they reside. Her experience and knowledge related to the complex emotional and physical needs of individuals dealing with infertility makes her an essential asset PVED.
As a previous recipient, Marna is uniquely qualified to provide caring and timely services. Marna is truly dedicated to compassionately guiding couples experiencing infertility through their treatment process.
Marna is joined by several dedicated and knowledgeable support staff that all work together clearly dedicated to see the success of PVED. These include clinical psychologists, reproductive endocrinologists, attorneys, as well as a talented business and public relations team.
Marna attended Eastern Oregon University and Portland State University majoring in Business, Psychology, Social Science, and is a member of the American Society of Reproductive Medicine (ASRM), the European Society of Human Reproduction and Embryology (ESHRE) and the Society for Assisted Reproductive Technology (SART). Marna, a writer, is married, has a son, and does some of her best thinking and creating atop of her John Deere tractor mowing and cultivating her back forty.
Final Post in Mini-Series: Thawing/Warming Embryos
 In the first two segments of this series, we discussed embryo grading and cryopreservation (1 – Do these embryos make the grade? and 2 – Embryo Cryopreservation: An Easy to Understand Review). This final segment in the series will examine the process of thawing or warming of embryos for transfer.
In the first two segments of this series, we discussed embryo grading and cryopreservation (1 – Do these embryos make the grade? and 2 – Embryo Cryopreservation: An Easy to Understand Review). This final segment in the series will examine the process of thawing or warming of embryos for transfer.
In Review…
Let’s begin with a short review of the previous blogs. You will recall that we discussed two methods of embryo cryopreservation, slow freezing and vitrification. Both methods require that the cells of the embryo have as much water removed as possible and that cryoprotectants, materials that protect the cells during freezing, be forced into those cells to reduce ice crystal formation. Slow freezing uses a controlled rate freezing instrument that slowly lowers the temperature to well below freezing to avoid the formation of ice crystals. Vitrification is a method in which embryos are plunged directly into liquid nitrogen and immediately turned into a glass-like substance, essentially cooling the embryos so quickly that ice crystals do not even have time to form.
Cryopreservation Storage Containers
One topic not covered in the freezing blog was the type of containers used to hold the embryos. Slow freezing, in most cases, uses straws or vials to hold the embryos in a small amount of solution called media. Vitrification uses many different storage devices to hold the tiny embryos, which cannot be seen without a microscope. These devices commonly include various types of straws, nylon loops or electron microscope grids, just to name a few.
How Often do the Cryopreserved Embryos Survive?
It is expected that about two-thirds of embryos cryopreserved via a slow freeze technique will survive thawing (Son and Tan, 2009) and the embryos that do not survive are most likely genetically abnormal. For embryos that are vitrified, the rapid cooling/warming process seems to work better, with about 80-90% of the embryos surviving. For this reason, vitrification is becoming more popular for cryopreserving both eggs and embryos.
How Many Embryos Should Be in Each Container?
Ideally only one or two embryos should be packed into each storage container. By packaging one embryo per container, the embryologist can thaw or warm only the exact number of embryos to be transferred without having any left over. For example, if a patient wants to have two thawed cryopreserved embryos transferred and they have a total of five embryos each frozen in separate containers, the embryologist will thaw/warm one container at a time until just two healthy embryos are recovered. If, however, the same five embryos were packaged in two containers with three embryos in one and two in another, the embryologist would probably first begin thawing/warming the container with three embryos. Should all of the embryos survive, there is one excess embryo that would need to be transferred, refrozen or discarded. While recent studies have indicated that freezing embryos a second time can result in a pregnancy, particularly when using vitrification, it is ideal to only thaw the exact number of embryos that we want to transfer and not one embryo more. (Kamasko, Y. et.al., 2009).
How are the Embryos Thawed/Warmed?
Ice crystal formation is also the enemy when thawing embryos, just as when cryopreserving them,. As embryos warm from -196°C (-321°F) toward 0°C (32°F), ice crystals may again form and damage the cells. We use the following techniques to rapidly warm/thaw both slow frozen and vitrified embryos in an attempt to avoid ice crystal formation:
- For embryos frozen by the slow freeze method, the straws or vials are removed from the storage tank and held at room temperature for 30-60 seconds, allowing the embryos to warm only slightly. The storage container is then plunged into 37°C water, completing the thawing process quickly enough so that the ice crystals don’t have a chance to form.
- Vitrified embryos are taken out of liquid nitrogen, with the container then plunged directly into warming media that is either at room temperature or 37°C, depending on the method used to vitrify the embryos. Once again, the ice crystals simply do not have enough time to form.
Once the embryos have been thawed/warmed, the cryoprotectants that replaced the water in the cells must be removed and balanced solutions placed back into the cells. To accomplish this, embryos are moved from small bath to small bath with varying concentrations of water and other substances. As the cryoprotectants are removed, the cells fill with water containing the nutrients and growth factors needed for cellular recovery. Once the embryos have been thawed/warmed, they are placed in the incubator in supporting culture media for two to twelve hours to allow the cells to continue equilibration prior to transfer. If we are thawing embryos that were frozen early, we may even grow the embryos for a few days so that we transfer the fewest healthy embryos we need to achieve a successful pregnancy.
Are There Other Factors That Influence the Survival of Cryopreserved Embryos?
While ice crystals play a big role in the survival of embryos, they are far from the only concern. Embryo survival rates vary for many reasons including;
- Embryo quality: Poor quality embryos freeze and thaw poorly. Poor early development of an embryo suggests that the embryo is in the process of dying and such embryos should probably not be cryopreserved.
- Freezing media and cryoprotectant “recipes“: Some recipes are potentially better than others and each laboratory has to find which works best. What works well for one, may not be the best for others.
- Laboratory techniques: Laboratories must have an excellent quality control program to assure they are following the steps involved in freezing/cooling and thawing/warming the cryopreserved embryos precisely.
- Embryologist variability: As with all techniques that involve humans, some seem to do a better job than others. There is no substitution for careful training and experience.
In Closing…
We hope this series has given you a glimpse of what is involved in grading, freezing/cooling and thawing/warming your precious embryos. Cryopreserved embryos give patients the option to build their families in a cost effective manner. Without cryopreservation, embryo donation would really not be possible.
Corey Burke, B.S, C.L.S.
Laboratory Supervisor
Embryo Donation International
CBurke@EmbryoDonation.com
References:
Kamasko, Y. et.al. The efficacy of the transfer of twice frozen-thawed embryos with the vitrification method. Fertil Steril 2009;91:383-386.
Son, W.Y., and Tan, S.L. Comparison between slow freezing and vitrification for human embryos. Expert Rev. Med. Devices 2009:6(1),1-7
Embryo Cryopreservation: An easy to understand review
Why are Embryos Cryopreserved?
Infertility patients invest so much time, effort, money and emotion into each IVF cycle. After the fresh embryos are transferred, about 30-40% of our patients will have excess high quality embryos available for freezing, also known as cryopreservation, to be used for future cycles. Frozen embryo transfers are a cost-effective way to pursue further treatment, regardless of the outcome of the fresh embryo transfer cycle.
Patients are often curious about the methods their laboratory uses to freeze their embryos. Cryopreservation is a delicate process requiring extensive expertise to prevent damage to the embryos during the freezing and thawing procedures.
Preventing Potential Damage During Freezing
There are three major types of injury that can occur to cells during the cryopreservation procedure (Jain JK, et al., 2006):
- (i) Exposure of cells to ice crystal formation during freezing and/or thawing;
- (ii) Damage to the embryos from the solutions used to prepare the embryos prior to freezing; and
- (iii) Damage to the embryos as water and electrolytes shift in and out of the cells that make up the embryos.
Damage from ice crystal formation is overcome by removing as much water from within the cells making up the early embryo and replacing it with fluid containing cryoprotectants. To remove the water, embryos are placed in a series of solutions using increasing concentrations of salt to draw the water out of the cells. Once this is accomplished, the embryos are then placed into additional solutions containing the cryoprotectants, which enter the cells taking the place of the water.
Removing the water from the cells is relatively simple while replacing the fluids with cryoprotectants is a bit more difficult and damage to the embryos can occur if not done correctly. Cryoprotectants can be toxic to the embryos, so the cells can only be exposed to them for a very short time before they are frozen. Once the cells have been filled with cryoprotectants, cryopreservation needs to take place fairly rapidly.
Slow Freezing and Vitrification Techniques
The two most common cryopreservation methods used to freeze embryos are slow freezing and vitrification. The slow
freezing method has been around for decades. Slow freezing involves loading the embryos into special straws or vials and placing them in a separate container, which is surrounded by a liquid nitrogen bath. The container controls the rate of temperature drop and freezes the embryos slowly over a few hours, preventing most ice crystal formation. The freezing device lowers the temperature gradually to -38° C. Once the embryos have reached this temperature, the vial or straw is plunged into a bath of liquid nitrogen to reach a final temperature -196°C (-321°F). They will remain at this temperature until they are thawed.
With vitrification, embryos are rapidly cooled to -196°C (-321°F) almost instantly. This instant cooling does not allow ice crystals to form. Vitrification comes from the Latin vitreum, meaning the transformation of a substance to glass. Please note the use of the word “cooling” is used rather than “freezing” when referring to vitrification. Freezing actually involves transitioning a liquid to a solid through crystallization. No crystals are actually formed during vitrification. To understand the difference better, take a look at an ice cube from your freezer. While it is mostly clear, you will notice it is somewhat cloudy due to ice crystal formation and not as clear as glass. Water that is vitrified appears absolutely clear because ice crystals never had the chance to form and a glass-like substance is created.
Vitrification still requires the replacement of water with cryoprotectants but uses a different recipe. In fact, the cryoprotectants used for vitrification are at a higher concentration and potentially even more toxic to the embryos. Once the embryos are exposed to the high concentration cryoprotectants, they must be frozen very rapidly. Whereas the slow freeze process takes place over hours, vitrification takes place over minutes.
Is One Cryopreservation Technique Better than the Next?
The process of embryo vitrification is relatively new compared to the slow freeze method; however, great advances have been made over the past decade.
There is evidence that vitrification is a slightly better system than slow freezing. In most studies, the embryo survival rates are better for vitrification than the slow freeze technique. A recent study by Kaskar, K. et.al, also showed that the pregnancy rates with vitrified/warmed embryos (64%) were significantly higher than those that were frozen/thawed by the slow freeze techniques (47%). With higher survival rates and higher implantation rates, vitrification is slowly replacing the slow freeze technique, especially for the storage of eggs and embryos.
Success with Re-vitrification
Vitrification may allow embryos to be warmed, re-vitrified, and warmed again with successful pregnancies reported. While the pregnancy rates are somewhat lower that that of embryos vitrified only once, the results are far better than embryos slow frozen, thawed, re-frozen, and thawed yet again. A 2007 study by Kamasko, Y. et.al, showed no significant differences in implantation rates between once vitrified embryos and twice vitrified embryos. The reason this is important is that embryos may be frozen/cooled in groups larger than we want to transfer. For example, if a patient succeeded with a twin pregnancy with two fresh embryos transferred and only wants one frozen embryo transferred for a future child, having two cryopreserved embryos survive that were stored in a single vial would present a problem. In this case, we would possibly transfer more embryos than we wanted to, discard the extra embryo (not at all desired) or refreeze/re-vitrify the extra embryo. Using vitrification, the embryo may indeed survive a second stage of warming for an additional pregnancy in the future.
Your Embryos Are Very Important
As embryologists, we take our responsibilities to care for your cryopreserved embryos very seriously so that you will have the opportunity to use them for future treatment cycles. Rest assured they will be available when you need them, having been cryopreserved with the most state-of-the-art techniques at our disposal.
Thawing/Warming Your Embryos
If you would like to learn about how embryos are thawed/warmed, be sure to watch for our upcoming blog on this topic!
References:
Kaskar, K., et.al. Comparison of clinical outcome of blasocyst vitrification with slow freezing and fresh embryo transfer. Fertil Steril 2010;94:113-114Jain JK, Paulson RJ. Oocyte cryopreservation. Fertil Steril 2006;86(Suppl 3):1037-46.
Kamasko, Y. et.al. The efficacy of the transfer of twice frozen-thawed embryos with the vitrification method. Fertil Steril 2009;91:383-386
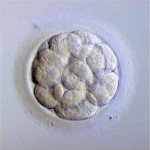
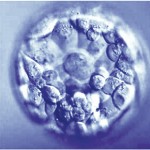
Do These Donated Embryos Make the Grade?
Embryo Grading Made Easy For Embryo Donors and Embryo Recipients
Part 1 of a 3 part mini-series by Corey Burke, B.S., C.L.S. & Laboratory Supervisor and Reproductive Endocrinologist Craig R. Sweet, M.D.
Embryo grading is an important factor for both donors and recipients. Potential embryo donors want to know if we will accept their embryos for donation since part of our decision is based on the grade of their embryos. Likewise, potential embryo recipients want to know how likely the donated embryos are to survive thawing and if they are of good enough quality to build their family. Part of our estimation of success depends on the grade of the embryos.
We wish this process could be easier since there is no standardized system used in all embryology laboratories to grade embryos. Furthermore, the grading of embryos is somewhat subjective so one embryologist may grade an embryo
somewhat differently than a colleague.
Embryo grading is an imperfect process; poorly graded embryos may occasionally result in ongoing pregnancies and beautiful looking embryos may not implant and grow. Poorer graded embryos will not necessarily result in an abnormal child; they simply seem to implant and grow less frequently.
So, the appearance and grading of an embryo is an imperfect estimate of the quality of the embryo as well as the embryo’s true potential. It is, however, the best way we have to visually estimate the implantation and live birth rate of a given embryo. We will now examine one of the more common methods used to grade embryos.
When are embryos graded and cryopreserved?
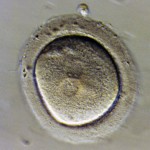
Embryos are usually graded and frozen at three specific stages with “Day 0” being the day of retrieval and fertilization:
| Age of Embryos | Day 1 | Day 3 | Day 5-6 |
| Common terminology | 2PN (pronuclear stage)
2 cell stage |
Embryos are 6-10 cells | Morula &
Blastocysts |
| Grading importance | Grading not available | Grading relatively important | Grading very important |
| How often these are sent to EDI? | Rarely sent | 40% of EDI embryos | 60% of embryos |
Embryos cryopreserved immediately after fertilization are confirmed early on Day 1 (the 2PN (pronuclear stage), but aren’t advanced enough to be consistently graded. Freezing embryos on Day 1 is quite infrequent unless we are certain there will not be an embryo transfer. Accordingly, these embryos are rarely sent to EDI for embryo donation.
Grading Day 3 Embryos
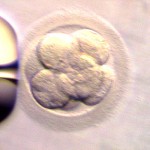
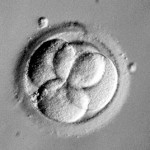
Day 3 embryos are graded on cell number, the amount of cellular fragmentation and the symmetry of the embryo.
Cell Number
Most Day 3 embryos will be comprised of 6-10 cells called blastomeres. Embryos with too few blastomeres may not be healthy, so we prefer at least 7-8 at this stage. Embryos with fewer cells may not be healthy growing very slowly or may have stopped growing entirely commonly called “cell block”.
Fragmentation
Fragments may be found in many of the embryos, which are “bits” of cells that break off from a blastomere. We prefer as little fragmentation as possible. Fragmentation is estimated as the percentage of fragmentation volume compared to the total embryo volume and is converted to a letter grade in the following way:
- 0 % = A
- 1-10% = B
- 11-25% = C
- >25% = D
A large amount of fragmentation may be caused by death of one or more of the blastomeres. The higher the fragmentation, the lower the quality of the embryo & letter grade and the less likely that the embryos will survive thawing. Embryos with high fragmentation rates implant less frequently when transferred fresh or when thawed.
Symmetry
Symmetry of the embryo refers to the shape of the individual blastomeres and the overall shape of the embryo. The blastomeres should all be very similar in size and generally round in shape. The scale used to grade symmetry is
- Perfect = A
- Moderately asymmetric = B
- Severely asymmetric = C
Severe blastomere asymmetry (i.e., large and small blastomeres in the same embryo) reflect nuclear/chromosomal and cytoplasmic problems suggesting the embryo is less healthy than desired. There is supporting evidence that blastomere symmetry is important and reflects overall health of the embryo. Interestingly, the overall symmetry of the embryo (round vs. oval) is of uncertain importance with some very “funny looking” embryos resulting in beautiful and healthy children.
Putting it All Together for Day 3 Embryos
For Day 3 embryos, the order of grading is the “number of cells (#c),” “fragmentation grade” and “symmetry grade”. For example:
- 7cAA = 7 cells with no significant fragmentation and perfect symmetry
- 8cBA = 8 cells with 1-10% fragmentation and perfect symmetry
- 6cBB = 6 cells with 1-10% fragmentation and moderate asymmetry.
Grading Day 5 Embryos
More advanced embryos are graded and potentially frozen on Day 5 or Day 6. These are generally described as morula or blastocysts.
Day 5 Morula Embryos
Morula embryos are difficult to grade as the cells combine, forming essentially a ball of cells that can’t really be categorized in any way other than descriptive terms:
- Morula (early)
- Compacting morula (more advanced)
While some facilities only occasionally cryopreserve Day 5 morula embryos, it is thought that the survival and implantation rates of these embryos may be slightly reduced but they are still quite reasonable, suggesting that they should not be discarded. Day 6 morulas are probably delayed in growth or may have stopped growing, may not be viable and are infrequently cryopreserved.
In order to balance the possible reduced implantation rates, it is common that more morula embryos are thawed and transferred in order to achieve success.
Day 5 Blastocyst Embryos
Day 5 blastocyst embryos are the most advanced embryos we see in IVF. These embryos are formed within 24 hour of actual implantation. Trying to grow embryos beyond this point is
technically difficult, as the embryos usually don’t survive. In addition, the window of time for implantation seemingly closes beyond Day 5 or early day 6. For example, transfer on Day 7 will rarely result in implantation. So, it simply makes more sense to transfer and/or freeze blastocysts rather than trying to grow them any further.
Along with descriptive measures, more objective grading is attempted through evaluation of the cellular expansion, the inner cell mass (which eventually becomes the fetus) and the quality of the outer cell mass called the trophectoderm (which eventually forms the membranes and placenta).
Expansion
As the embryo advances in growth, a cavity called the blastocoel fills with fluid. As the cells continue to divide and the fluid collects, the embryo expands and eventually escapes its outer covering called the zona pellucida. The blastomeres continue to group together wherein the individual cell cannot be counted. As the Day 5 embryo expands, differentiates and escapes the outer zona pellucida, the grade increases numerically from 1-5.
| Grade | Description | Physiology |
| 1 | Early Blastocyst | Starting to form a fluid-filled space in the middle (Blastocoel). Grading the embryo is difficult here. |
| 2 | Full Blastocyst | Blastocoel forms and inner cell mass is now distinguishable. Grading can be done from this point forward. |
| 3 | Expanded Blastocyst | Blastocyst is starting to expand in size thinning the outer covering, the zona pellucida |
| 4 | Hatching Blastocyst | Blastocyst is starting to hatch out of the zona pellucida. |
| 5 | Hatched Blastocyst | Blastocyst is fully hatched and now ready for implantation into the uterine wall. |
Inner Cell Mass (ICM)
As the blastomeres compact to form the inner cell mass (ICM), this early fetal tissue is graded on a A-D scale:
| ICM Grade | Description |
| A | ICM with total compaction |
| B | ICM still compacting |
| C | Reduced ICM |
| D | Poor with dying cells |
Trophectoderm (TE)
The outer cells of the trophectoderm (TE) also reflect the overall health of the embryo and are graded in an A-D scale.
| TE Grade | Description |
| A | Numerous cells forming cohesive layer |
| B | Few but healthy large cells forming a loose epithelium |
| C | Few cells present often with asymmetric distribution |
| D | Poor with degenerating/dying cells |
Putting it All Together for Day 5 Embryos
For Day 5 embryos, the order of grading is “expansion,” “inner cell mass” and “trophectoderm.” For example:
- 1 = Early blastocyst is unable to be easily graded with respect to ICM or TE as these haven’t separated well enough yet.
- 2BB = Blastocyst with partial ICM compaction with loose, large trophectoderm cells
- 3AB = Expanding blastocyst with total compaction of ICM and with loose, large trophectoderm cells
- 4AA = Hatching blastocyst with excellent ICM & trophectoderm cell layers
- 5AA = Fully hatched blastocyst with excellent ICM & trophectoderm cell layers
Day 5 embryos that are graded 4AA and 5AA are some of our favorite embryos.
Summary Comments
Embryology laboratories strive to grow the healthiest embryos they can. Over time, they have adapted different grading techniques so the laboratories can communicate the quality of the embryos to physicians, patients and each other. Not all beautiful embryos will implant or produce a healthy child but they seem to do so more often than others. Not all poorly developing embryos will fail to implant and produce a healthy child but most do not result in live offspring.
At EDI, we try to only accept embryos that are likely to implant so embryos with less than a B rating for any category are infrequently accepted. This is done to assure our recipients that all of the donated embryos we offer are of the highest quality and provided the greatest chance for a successful pregnancy.
This is only one piece of the puzzle as many other factors influence the likelihood for success. Dr. Sweet will cover this topic in the separate blog within the next couple of months.
Also stay tuned to the upcoming blogs regarding how embryos are frozen and thawed and what techniques seem to work the best.
While embryo grading is not a perfect system, we use it to try to predict the overall quality of the embryos and their potential to survive thaw, grow and build a recipient’s family.
Corey Burke. B.S., C.L.S.
Laboratory Supervisor
CBurke@EmbryoDonation.com
Craig R. Sweet, M.D.
Reproductive Endocrinologist
Info@EmbryoDonation.com
References
Racowsky C, Vernon M, Mayer J, Ball GD, Behr B, Pomeroy KO, Wininger D, Gibbons W, Conaghan J, Stern JE. Standardization of grading embryo morphology. Fertil Steril. 2010 Aug;94(3):1152-3.
New Mini-Series on How Embryos are Processed
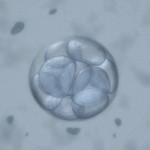 Beginning next week, we’ll be publishing a mini-series on how embryos are processed in the laboratory. Written by Corey Burke, B.S., C.L.S. & Laboratory Supervisor and Reproductive Endocrinologist Craig R. Sweet, M.D., the three-part series will cover how embryos are initially graded, frozen and finally thawed. The information is designed for patients and written in an easy to understand style.
Beginning next week, we’ll be publishing a mini-series on how embryos are processed in the laboratory. Written by Corey Burke, B.S., C.L.S. & Laboratory Supervisor and Reproductive Endocrinologist Craig R. Sweet, M.D., the three-part series will cover how embryos are initially graded, frozen and finally thawed. The information is designed for patients and written in an easy to understand style.
We hope this will be an informative series, answering many common questions alike. If you have additional questions, please feel free to post them as comments here, or after the specific blog post.
Thank you for reading and stay tuned for detailed embryo grading, freezing and thawing information starting with the first series post on Tuesday, January 10th.
–Embryo Donation International